One of these compounds is Aspartame (Nutrasweet -- the stuff in the blue packets), and the other is Methamphetamine (Meth, crystal).
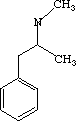 space between the two space between the two 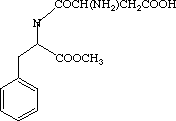 |
One of these compounds is Acesulfame (that European stuff in Pepsi One), and the other is Barbituric Acid (Barbs, downers).
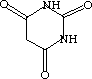 space between the two space between the two 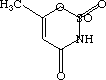 |
One of these compounds is Cyclamic Acid (Cyclamates -- used in sugar-free Kool-Aid and "Fizzies" back in the 1960's), and the other is Ammate (a weed killer).
NH4NH2SO3 space between the two C6H11NH2SO3One of these compounds is Sucralose (Splenda -- the stuff in the yellow packets), and the other is Dioxin (the reason Times Beach, Mo. ceased to be).
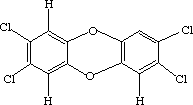 space between the two space between the two 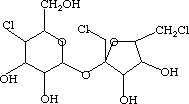 |
One of these compounds is Saccharin (Sweet 'n' Low -- the stuff in the pink packets), and the other is Acyclovir (Zovirax -- kills herpes viruses).
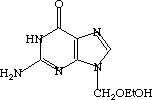 space between the two space between the two 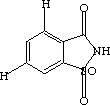 |
Disclaimer: these molecular structures are not necessarily the way the compounds are normally represented. They may have been tweaked to make them look like other compounds which they may or may not resemble from toxicological and chemical standpoints. The perpetrator of this page is a Chemistry teacher with a strange sense of humor who is having way too much fun with ChemDraw. Scroll down to find out which structures represent sweeteners and which represent toxic, nasty things.
..
.
.
.
.
.
.
.